Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
Anode Material Could Help Lithium-ion Batteries Last Longer
Battery Materials: Tiny porous germanium particles hold promise for high-energy batteries
by Prachi Patel
December 16, 2011

Electronic gadgets and electric vehicles would run longer if lithium-ion batteries could store more energy. Such high-energy batteries would need to hold more ions, or charge, than do batteries equipped with today’s standard graphite electrodes. Researchers have now made electrodes out of porous germanium that hold more than three times as much charge as their graphite counterparts do (J. Am. Chem. Soc., DOI: 10.1021/ja208880f).
In theory, germanium and silicon can pack, respectively, about five and 10 times as much charge per weight as graphite does. Researchers have developed high-capacity battery electrodes using germanium nanotubes and silicon nanowires. But their capacity quickly fades after they’re recharged 250 to 400 times--not enough for a consumer electronics battery, which typically goes through at least 500 recharge cycles. These charging cycles break down battery electrodes when the electrode material swells and shrinks as it absorbs and releases lithium ions.
Weiqiang Han, a materials scientist at Brookhaven National Laboratory, turned to highly porous particles of germanium oxide because the material can hold more charge per weight than pure germanium does, and it doesn’t swell as much. He and his colleagues reduced germanium dioxide to produce a compound/material called germanium suboxide, which has a higher germanium-to-oxygen ratio than the dioxide does.
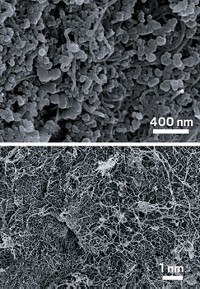
Using scanning electron microscopy and transmission electron microscopy, the researchers found that the germanium suboxide powder consists of porous, micrometer-sized grains, which themselves are made of 3.7-nm-wide particles clustered together. Using X-ray diffraction, the researchers showed that the material lacks a crystalline structure.
Finally, the researchers built a battery anode by coating the germanium suboxide powder on copper foil. They found that the anode can charge and discharge 600 times.
The material’s high performance stems from its small size, porosity, and lack of regular structure, Han says. Unlike the regular crystals of silicon and germanium, the new material’s nanoparticles, says Han, are too small to break down under mechanical stress. The pores in the micrometer grains also give the nanoparticles space to swell, while the amorphous structure, unlike crystals, can handle expansion and contraction without cracking, he explains.
Yi Cui, a materials science and engineering professor at Stanford University, calls the study “exciting and promising,” adding that manufacturing the material should be easy at a commercial scale. “The only caveat with germanium is it’s too expensive and not as widely available as silicon,” he says.
But Jianyu Huang, a materials physicist at Sandia National Laboratories says that germanium is still promising for battery anodes because lithium ions move through it 400 times faster than they do in silicon. “You can charge and discharge much faster,” he says. He calls the new material’s high capacity and long cycle life “very impressive.”



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter