Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Infectious disease
Unraveling Ebola
Scientists are deciphering the virus’s inner workings in an effort to develop effective drugs
by Lisa M. Jarvis , Bethany Halford
November 26, 2014
| A version of this story appeared in
Volume 92, Issue 48

After Craig Spencer, the American doctor who contracted Ebola while volunteering in Guinea, walked out of a New York City hospital last month, national anxiety over Ebola seemed to dissipate. Days earlier, quarantines had been lifted on those who had interacted with him.
America relaxed, but the epidemic rages on in West Africa. Even as evidence mounts that the outbreak is abating in some regions, flare-ups in other areas make experts reluctant to say the epidemic is under control. To date, more than 15,300 people have contracted the virus in Sierra Leone, Guinea, and Liberia. Of them, nearly 5,500 have died.
Those numbers weigh heavily on researchers who have dedicated their careers to understanding and developing drugs against the Ebola virus. Their labs are far away from the battlefield, but they are still on the front lines. Finding effective treatments could do more than just quell the current outbreak. Critically, it could prevent future ones.
“The pressure is tremendous,” says Travis K. Warren, a scientist at the U.S. Army Medical Research Institute of Infectious Diseases (USAMRIID), who has worked on several of the Ebola treatments that have been tested on American and European patients. USAMRIID, like many other labs with expertise in Ebola, has set aside all other work to determine as quickly as possible which treatments are most promising, to gather more information about how the virus interacts with different drugs, and to assess whether any other untested molecules might also be effective against the virus.
That work is being done at a breathtaking pace. Some programs have gone from the petri dish to tests in monkeys in the span of a month, says Sina Bavari, chief of molecular and translational sciences at USAMRIID. “We’re doing studies on top of studies to just make sure that we can appropriately respond to the outbreak,” Bavari adds.
But scientists have been trying to conquer Ebola for almost 40 years. The question lingers: Why has it been so hard to develop a drug to take down the virus?
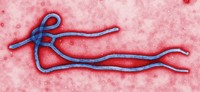
Researchers point to a deceptively simple system—each Ebola virus particle is little more than a single strand of RNA coated with proteins and encased in two layers of lipids—that does a bang-up job of fooling its host.
“I like to think of the virus as a guided missile,” says Kartik Chandran, a microbiologist at Albert Einstein College of Medicine. “It’s got a payload and a delivery system.”
That RNA payload is surprisingly small. The Ebola virus is endowed with just seven genes that synthesize a mere eight proteins. By contrast, humans have more than 20,000 genes.
But don’t let the simplicity fool you. Each protein made by the Ebola virus is as multifunctional as a Swiss Army knife. And the virus also will beg, borrow, and steal to survive: USAMRIID researchers found that the virus uses nearly 70 proteins from its host. It is so resourceful that just a few particles—possibly even one—can be lethal.
To find starting points for drug development, researchers have spent years trying to unravel the form and function of the proteins the Ebola virus makes as well as where and how they interact with the proteins of its human hosts. They’ve tried a range of therapeutic approaches—antibodies, small molecules, RNAi-based drugs—to prevent it from replicating.
The drugs attempt to hit the virus at various stages as it settles into its host. Knock out the right protein, block the right landing spot on the host, and perhaps the virus can be stopped in its tracks. Keep it from copying its genetic blueprint, or from building the proteins it needs to multiply, and maybe it will be too crippled to survive. Researchers are trying all of these.
HOME IN ITS HOST
After it finds its way into the body through, for example, a drop of blood, the first thing the Ebola virus needs to do to survive is put down an anchor. That anchor is the Ebola virus glycoprotein, a huge, heavily glycosylated molecule that attaches to the surface of the cell, setting the stage for the virus to sneak inside, unleash its genetic payload, and start replicating.
For years, researchers have sought some way to keep the virus adrift. One strategy now being pursued in the clinic is to use convalescent serum, which comes from the blood of Ebola survivors and provides antibodies that fight the virus. Another is to develop antibodies directed against the glycoprotein. Indeed, ZMapp, the “secret serum” given to Kent Brantly and Nancy Writebol, the first two people to be treated in the U.S. for Ebola, was a mixture of three antibodies that bind to the protein and keep it from anchoring inside a host cell.
Doctors don’t know whether ZMapp, which was developed by the tiny biotech firm Mapp Biopharmaceutical, actually helped Brantly and Writebol overcome the virus, or if their survival was instead due to the intensive care they received at Emory University Hospital. But researchers think an antibody cocktail that targets the Ebola glycoprotein is likely the best hope right now for a treatment that could work after someone is symptomatic.
Amazingly, the antibody cocktail didn’t exist a year ago, according to Erica Ollmann Saphire, a structural biologist at Scripps Research Institute in La Jolla, Calif., whose lab focuses on viruses.
The mixture was the result of painstaking work to understand the structure and function of the Ebola glycoprotein. A breakthrough came in 2008, when Ollmann Saphire’s lab solved the structure of the glycoprotein bound to an antibody from an Ebola survivor. The crystal structure revealed several floppy sugar molecules that form a canopy over the portion of the glycoprotein responsible for binding inside the host cell.
Once the glycoprotein is inside the endosome—a cellular sorting compartment that typically breaks down proteins for recycling—one of the host’s enzymes, called cathepsin, snips off the glycan shield. What’s revealed is the portion of the protein that attaches to the host.
That canopied structure not only provided starting points for drug discovery but also explained why earlier efforts to develop antibodies against the virus failed: Some antibodies target parts of the glycoprotein that are lopped off in the endosome, leaving the critical core of the molecule free to do its business.
Right now, researchers developing therapeutics against the glycoprotein don’t fully understand why the three antibodies in ZMapp seem to work—or even if all three are required.
Because of the elaborate glycan shield and the speed of replication, among other factors, more than one antibody is likely required to do the trick, Ollmann Saphire says. So far, though, the only evidence to support that hypothesis comes from studies of KZ52, an antibody retrieved from the bone marrow of a survivor of a 1995 Ebola outbreak in Zaire. Although KZ52 cured mice with Ebola, it did not save monkeys with the disease.
It turns out that KZ52 blocks most, but not all, of the viral particles, Ollmann Saphire explains. And just a few, or maybe even one, of them can be deadly. “It’s a really tall order to ask one monoclonal antibody to scrub out every viral particle,” she says.
Since the outbreak, Ollmann Saphire’s lab has also been working around the clock to understand where the antibodies in ZMapp bind. Last month, she and fellow Scripps scientist Andrew Ward showed that two of them seem to be going after the same site on the glycoprotein. The researchers now wonder whether the cocktail being used makes the most sense or if it would be better to choose an antibody that blocks a new site on the virus.
WELCOME TO THE ENDOSOME
Pruning the glycoprotein’s sugary shield reveals what scientists think is another promising avenue for drug discovery. Sitting on that pared-down glycoprotein is a receptor that binds to a membrane protein in the host cell called Niemann-Pick C1. Normally, this protein’s job is to shuttle cholesterol within the cell. Mutations to the gene that makes the protein result in the rare, fatal Niemann-Pick disease.
Ebola needs the Niemann-Pick C1 protein to infect a host cell, Einstein’s Chandran says. Indeed, cells from patients with Niemann-Pick type C1 disease resist Ebola infection, something scientists learned only a few years ago. If scientists can find small-molecule inhibitors that block the virus from binding this protein, they can stop the infection process at an early stage.
“The advantage of these types of inhibitors is that they should work against all strains of the virus,” Chandran explains, because they work on the host cell rather than the virus itself. A number of small molecules have been shown to prevent Ebola infection in tissue cultures via this mechanism, but none is a viable drug candidate yet.
Once the virus has entered the endosome, it hunkers down for transcription and replication. Essentially, the virus is turning the host cells into its own little protein factory.
Transcription kicks off the event by making copies of all of the instructions for the virus’s RNA, a process mediated by viral polymerase. Researchers are now exploring whether small molecules developed for other viruses might be able to throw a wrench in the viral xeroxing.
Two drugs in particular seem to have potential for mucking up transcription and viral RNA replication in Ebola: BioCryst Pharmaceuticals’ BCX4430, which is in preclinical testing, and Fujifilm’s favipiravir, a flu drug already on the market in Japan as Avigan.
Scientists think that when BCX4430 and favipiravir enter cells, enzymes blithely tack on ribose units and phosphate groups as needed with the result that the drugs are disguised as RNA nucleotide components. The viral polymerase incorrectly identifies the decorated drugs as RNA building blocks and starts incorporating them into the copies of RNA that it’s making to replicate the virus. “They’re sort of like poisons that prevent viral genomes from being copied,” Chandran says.
BCX4430, for example, is thought to look to viral RNA polymerases like the purine base adenosine. “It creates a distortion in the new RNA molecule such that only one or two additional bases are able to be added before the polymerase realizes a mistake has been made and terminates that RNA synthesis reaction,” explains USAMRIID’s Warren, who has worked with BCX4430.
The challenge to designing such antivirals is to make them able to trick the viral polymerase without also fooling the host cell’s polymerases. If the host polymerases can’t make the distinction, Warren says, the drug will almost certainly be toxic.
Scientists think that favipiravir works similarly to BCX4430. Warren tells C&EN that scientists at USAMRIID recently tested favipiravir in monkeys and found it to be effective at treating the Ebola virus. Those results have not yet been published.
The drug was given to infected patients in France, Spain, and Germany, who later recovered, and the nonprofit Doctors Without Borders announced that it and partner organizations will begin clinical trials of favipiravir in West Africa this month. Since the drug has already been approved to treat the flu, the thinking is that, even if it turns out to be ineffective, it won’t do any harm.
Doctors Without Borders will also be conducting clinical trials of the antiviral brincidofovir in West Africa this month. Brincidofovir, which is made by the biotech firm Chimerix, is currently in Phase III trials to treat both adenovirus and cytomegalovirus. Three of the Ebola patients treated in the U.S. have been given brincidofovir. Two survived, but it is not known whether they actually benefited from the drug.
The compound works by inhibiting viral DNA polymerase. Ebola doesn’t have DNA—it has RNA—making any effectiveness of the drug against Ebola something of a mystery. Brincidofovir is actually a prodrug of cidofovir, a compound that’s been shown to be ineffective against RNA viruses, Chandran points out. But when the Centers for Disease Control & Prevention screened brincidofovir in a cell culture of human Ebola infection, it turned out to be remarkably effective.
Advertisement
“It clearly inhibits the virus in cells, but lots of things inhibit the virus in cells. Half of the Sigma catalog does,” Chandran says. “The question that really needs to be answered is, Is this actually effective in a therapeutic setting? The fact is that we don’t really know how it works or if it works.”
CAPTURING TRANSCRIPTS
Whereas antivirals disrupt transcription altogether, other treatments are trying to stop snippets of RNA from doing their job. Two companies are using RNAi-based technologies—either single- or double-stranded RNA—to prevent key proteins from being manufactured.
Sarepta Therapeutics has spent more than a decade working on the Ebola virus, with much of that research done in partnership with USAMRIID. Because designing oligonucleotides to block RNA transcripts, or copies, is fairly straightforward, the company took an empirical approach to figuring out which ones would make the most sense as a drug.
“Basically, because there are only seven genes, we targeted all seven,” says Patrick Iversen, a Sarepta scientist who has worked on the company’s Ebola program since its inception in the early 2000s.
The company skipped testing its oligonucleotides in cell culture and went straight to putting them in animals, conducting dozens of studies in mice to understand the effects of blocking the production of individual proteins and protein combinations.
The most promising targets were the transcripts for three proteins: VP24, VP35, and L. In the early 2000s, conventional wisdom among virologists was that L, which contains the genetic code for viral polymerase, might be the most vulnerable spot in the Ebola genome.
But Sarepta found that L poses a problem. It’s the first gene copied during transcription, and and as a result, more copies of L tend to be made than are made of the other proteins. It was too tough to take out all of it. “Even if you knock down 90%, you can still produce quite a bit of virus,” Iversen notes.
So Sarepta focused instead on VP24 and VP35. Each of those proteins plays dual roles: Together, they help form the nucleocapsid, the carefully arranged protein layer surrounding the virus’s genetic material, and they also interfere with the body’s immune response to the virus.
Sarepta put oligos targeting each transcript individually, as well as a combination of the two, into guinea pigs infected with Ebola. “We found in a real clean and clear study—and to our surprise—that you only needed VP24,” USAMRIID’s Bavari says. The resulting drug was AVI-7537, which blocks the transcript for VP24.
More recent research has helped explain why VP24 turned out to be the best target for Sarepta’s antisense program, Iversen says. Scientists have found that VP24 is able to disrupt the activity of a signaling protein called STAT1, which turns on a host of genes that regulate interferons, the body’s natural defense against viruses. “Essentially the virus is in some measure crippling the immune response,” Iversen says.
AVI-7537 showed promise in monkeys and in an early human clinical trial, but in 2012, in the face of budget cutbacks, the Department of Defense stopped funding the project. The company is now trying to revive it. “We are actively pursuing funding opportunities both within the U.S. and externally with philanthropic foundations,” says Michael Wong, Sarepta’s senior medical director for infectious diseases.
Although Sarepta’s funding was cut, the government did continue to support an RNA-based therapeutic from Tekmira Pharmaceuticals. That project uses small interfering RNA (siRNA), a class of double-stranded RNA, to target the transcripts of several key proteins.
Tekmira’s early Ebola treatment used three different siRNAs to block the production of VP24, VP35, and L. In 2010, the company published a study showing the trio worked well in monkeys with Ebola, but the dosage needed to be effective in humans was too high to be practical. It was also administered intravenously, and the military wanted a format that could be more easily transported.
Tekmira reworked the drug, known as TKM-Ebola, into a powdered formulation and pared back the transcripts being targeted to VP35 and L. The company says the drug is now 30 times more potent in monkeys.
Tekmira declined to be interviewed for this story, but at an October symposium held as part of the University of British Columbia’s Neglected Global Diseases Initiative, the firm said that a drug with its updated siRNA had been used to treat infected patients. It also says that the virus raging through Guinea has changed enough to render the original therapy less effective there, and it has now developed another drug, TKM-Guinea, in response. Tekmira highlighted the ease with which it can keep up with changes to the virus by adjusting the sequence of its siRNA.

PRACTICAL REALITIES
All of the treatments in development are so new that predicting which will work best is anybody’s guess. Ask someone who has been on the ground treating Ebola patients in West Africa and you’re likely to hear that the most practical drug—a pill based on an easy-to-manufacture small molecule—is not necessarily the most promising.
Armand Sprecher, a public health specialist with Doctors Without Borders, says the ideal drug is effective after someone is showing symptoms of infection. So far, only the monoclonal antibodies—which need to be stored in a freezer and have to be given intravenously—appear to fit that bill.
A race is now on to figure out which treatment is most effective, and U.S. and European regulatory agencies, the World Health Organization, and nonprofits are working together to design the right clinical studies. “Clearly in this setting, we need to get to clinical trial designs that will get to the right answer quickly,” Edward M. Cox, director of the U.S. Food & Drug Administration’s Office of Antimicrobial Products, told reporters last month during the American Society of Tropical Medicine & Hygiene’s annual meeting.
FDA advocates randomized studies in which every patient gets supportive care and a subset of patients gets an Ebola treatment. But such studies also will have multiple checkpoints built in to allow data to be analyzed for signs of a treatment’s efficacy; if an investigational drug looks good, even at an early checkpoint, everyone in the study will be given it. Cox believes this novel trial design will “allow a winner to be declared very early.”
Other practical realities will influence what the “right” drug is for this outbreak. “To a certain extent, a lot depends on the right drug being available early enough for us to be able to study it before the outbreak ends,” Sprecher says. ZMapp, for example, is grown in tobacco plants, a process that takes months.
If enough material for a clinical study isn’t available until later in 2015, the outbreak may have abated and there might not be enough sick people to test. That sounds like a good problem to have, but everyone from basic researchers to relief groups to government agencies wants to figure out an answer to avoid future crises. “It would be really nice to not have to go through this again,” Sprecher says.
Researchers, meanwhile, stress the need for ongoing funding to support Ebola research, even after this outbreak has died down. “If we want these therapies and vaccines, we have to put in the legwork and do it over a long period of time, when nobody is watching, not just when there is public interest,” Einstein’s Chandran says. “Right now there is a clamoring, ‘Give me everything you have. Open your cupboards.’ It doesn’t work like that. It takes years to develop a drug.”





Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter