Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Physical Chemistry
Aromaticity For All
Chemists argue over how the sacred concept of aromaticity should be invoked
by Stephen K. Ritter
February 23, 2015
| A version of this story appeared in
Volume 93, Issue 8

Read Boldyrev and Wang’s response to Hoffmann’s article, a rebuttal by Hoffmann, and perspectives from other researchers, then add your thoughts at http://cenm.ag/aromatic.
Aromaticity is one of chemistry’s oldest concepts to describe the behavior of molecules. Chemists created it 150 years ago to help visualize and explain the bonding, structure, and reactivity of benzene.
Many chemists today hold aromaticity sacred and defend using the concept only for benzene, its many derivatives, and related heterocyclic compounds. Others believe the concept is so fundamental to chemistry that it should also be used to help explain the bonding, structure, and reactivity in any type of molecule, including fleeting inorganic compounds.
This repurposing of aromaticity is, to some traditionalists, the equivalent of claim jumping and is asking for a fight. In fact, theoretical chemist and Nobel Laureate Roald Hoffmann of Cornell University threw down a gauntlet last month when he published a historical perspective on the discovery and understanding of aromaticity in his regular column in the magazine American Scientist (2015, DOI: 10.1511/2015.112.18). In the article, Hoffmann lamented that some chemists had lately been stepping out of bounds by using aromaticity to describe bonding in planar and three-dimensional inorganic compounds.
Hoffmann referred to these chemists as “purveyors of hype.” Those purveyors, namely Alexander I. Boldyrev of Utah State University and Lai-Sheng Wang of Brown University, took Hoffmann’s comments as a terse indictment of their work and picked up his gauntlet, defending their usage of aromaticity. Their written response, a rebuttal by Hoffmann, and commentaries from other researchers in the field are available in full on C&EN Online (http://cenm.ag/aromatic).
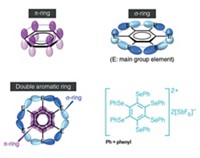
Historically, chemists have viewed the characteristic stability of aromatic compounds as stemming from delocalized electrons in π bonds about the ring. These bonds form from molecular orbitals that overlap at two points, above and below the plane of the ring, and they must contain by definition 4n + 2 π electrons. But Boldyrev, Wang, and others suggest that compounds can also exhibit aromaticity involving other bond types, such as σ and δ bonds, which have a single point and four points of orbital overlap, respectively. They also suggest there can be mixtures of different types of aromaticity in the same molecule.
Hoffmann writes that “an inflation of hype” threatens the traditional, “beautiful” concept of aromaticity. “A century and a half after the remarkable suggestion of the cyclic structure of benzene, the conceptual value of aromaticity—so useful, so chemical—is in a way dissolving in that hype.”
Hoffmann says that many of the compounds produced by Boldyrev, Wang, and others “have precious little chance” of being made on a significant scale. As part of his argument, he puts forward two stringent criteria for aromatic molecules: They should not be overly reactive, and they should be bench-stable or bottleable.
Boldyrev and Wang have been chipping away at that confining notion over the past 15 years. Boldyrev is a computational chemist whose group determines whether imagined molecules are physically plausible, and Wang is an experimentalist whose group attempts to make them. The researchers generate a molecular beam of these species by vaporizing a disk containing the elements of interest with a laser, selecting out the target molecules via mass spectrometry, and then confirming their existence in the gas phase with photoelectron spectroscopy.
In 2001, Boldyrev and Wang reported the square-planar all-metal aromatic cluster Al42–. They contend that Al42– fulfills all the criteria for aromaticity, including 4n + 2 (n = 0) π electrons, equivalent Al–Al bonds, and high resonance stabilization energy. “If it walks like a duck, quacks like a duck, and looks like a duck, it must be a duck!” Boldyrev and Wang write in their response. “We do not see any ‘hype’ in the characterization of Al42– as being aromatic.”
Boldyrev and Wang subsequently made a series of planar boron clusters that they compare with polyaromatic hydrocarbons. For example, they argue that the wheel-shaped molecule B9– is aromatic like benzene. Because some of the compound’s delocalized electrons are σ electrons and have the same bonding pattern as the π electrons, the researchers conclude that the molecules exhibit σ and π aromaticity, so-called double aromaticity.
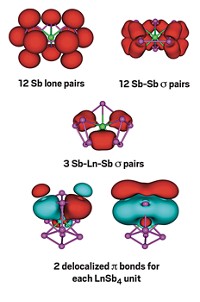
Other manifestations of nontraditional aromaticity include the σ aromatic PtZnH5– cluster reported by the University of California, Los Angeles’s Anastassia N. Alexandrova and coworkers, the δ aromatic Ta3O3– cluster reported by the Boldyrev and Wang team, and a 3-D aromatic fullerene analog B40– reported by Wang and other collaborators.
It’s true that none of these species have been “bottled.” But before Boldyrev and Wang got started, chemists had reported nontraditional compounds that had aromatic properties and could be isolated. For example, some researchers had been making metallabenzenes—predicted earlier by Hoffmann and his colleagues—in which a metal replaces a C–H group in the benzene ring. In addition, Gregory H. Robinson and coworkers at the University of Georgia provided the first solid evidence for all-metal aromaticity in 1995 when they prepared a gallium complex with a triangular Ga3 core.
“Aromaticity has in recent years become an important and powerful unifying concept to describe the stability and bonding in many chemical species beyond organic chemistry, including inorganic cations, anions, radicals, and clusters,” Boldyrev says. He doesn’t think the reactivity or the inability to bottle the species should preclude them from being labeled aromatic.
Hoffmann doesn’t deny that the molecules exist and can be studied. “But to label them as aromatic, with the 150-year-old history of thermodynamic stability, kinetic persistence, and chemical reactivity associated with that concept, as well as the 20th-century correlates we’ve added [for example, aromaticity in spherical molecules such as fullerenes], is to me—and I think to many—a stretch,” he says.
Hoffmann threw down a second gauntlet by offering “a good bottle of New York state Riesling” to anyone who prepares milligram quantities of the newfangled compounds. Hoffmann says he would even accept as convincing evidence a study on the dimerization or polymerization of one of the molecules or a study showing that it is persistent under ambient conditions.
Wang responded that a bottle of Riesling isn’t enough incentive to drop everything to make a vial of Na2Al4. He might do it for a bottle of champagne.
Gernot Frenking, a computational chemist at Philipps University, in Marburg, Germany, shares Hoffmann’s view on these new species. “There was a time when I was wondering if there were molecules left that do not exhibit some kind of aromaticity.” At the same time, Frenking doesn’t fault Boldyrev and Wang for picking up on the concept and showing that it can be useful for providing new understanding of the nature of matter.
“I personally do not care whether Al42– can be considered aromatic or not,” says theoretical chemist Dage Sundholm of the University of Helsinki, in Finland. “Aromaticity is just a name. What is more important to me is that molecules such as Al42– are formed in molecular beam experiments and how we can explain why this species is more abundant in the beam than other negatively charged aluminum clusters.
“I agree there is aromaticity hype,” Sundholm continues. “However, that will disappear in the future when we have invented better and more reliable methods to determine the degree of aromaticity, or whatever we agree to call it.”
Read Boldyrev and Wang’s response to Hoffmann’s article, a rebuttal by Hoffmann, and perspectives from other researchers, then add your thoughts at http://cenm.ag/aromatic.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter