Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Neuroscience
The Pesticide Connection
Medical mystery jump-starts investigation of the link between Parkinson’s disease and crop-protecting chemicals
by Lauren K. Wolf
November 25, 2013
| A version of this story appeared in
Volume 91, Issue 47

The rats in a room at the University of Pittsburgh regularly get hit with doses of pesticide. But the researchers in J. Timothy Greenamyre’s lab don’t expose the rodents because of an infestation problem. They give the neurotoxin to the animals to learn more about Parkinson’s disease.
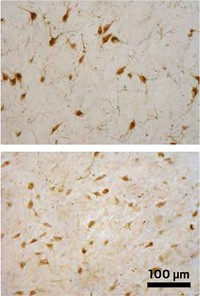
After receiving a low daily dose of the pesticide rotenone for a week or two, rats in Greenamyre’s lab begin to lose mobility in ways similar to Parkinson’s patients. The rodents move at a glacial pace, they have trouble keeping their balance, and their limbs become impossibly stiff. Even the animals’ brains develop classic signs of the nervous system disorder: Nerve cells in a region called the substantia nigra accumulate clumps of the protein α-synuclein and die.
It’s not unusual to use animal models such as these to probe the molecular causes of Parkinson’s, which affects 7 million to 10 million people worldwide, and to test treatments. But their use also raises a question: If a chemical gives lab rats Parkinson’s symptoms, might it do the same to humans exposed in the real world?
COVER: Exposure to the pesticides paraquat (top left) and rotenone, shown with an artistic rendition of a nerve cell junction, has been associated with the development of Parkinson’s. Credit: Shutterstock/C&EN
To download a pdf of this article, visit http://cenm.ag/pesticides.
A number of population studies have reported that people living in rural areas and people who work with pesticides have a higher incidence of Parkinson’s disease. In the past five years, some investigations have even associated the use of specific pesticides, such as rotenone, with a higher risk of having the disorder. And most recently, researchers have been investigating whether specific people might be vulnerable to pesticides because of their genes: People are at greater risk if they have mutated versions of enzymes or protein pumps that protect cells against damaging substances.
On the basis of these results, many in academia say the link between pesticides and Parkinson’s is clear. But others, including pesticide industry representatives, argue that these studies are fraught with bias, and other research has not shown a Parkinson’s-pesticide tie-in.
One thing these two camps agree upon, though, is that the story of how scientists connected pesticides to Parkinson’s is a medical mystery worthy of the big screen.
THE CASE OF THE FROZEN ADDICTS
In the summer of 1982, a 42-year-old man named George Carillo was transported from a nearby prison to Santa Clara Valley Medical Center, in San Jose, Calif. Unable to speak and rigid from head to toe, Carillo perplexed many of the center’s doctors. At a loss, they gave him an initial diagnosis of catatonic schizophrenia.
After spending some time with Carillo, the facility’s head neurologist, J. William Langston, came to a different conclusion. He felt that the paralyzed man’s symptoms more closely aligned with advanced Parkinson’s disease. The trouble with this diagnosis was that Parkinson’s doesn’t take hold of a person overnight—which seemed to be the case with Carillo. Complicating matters was the disturbing discovery that Carillo’s girlfriend, 30-year-old Juanita Lopez, was “frozen” too. Surely this wasn’t coincidence, but then again, Parkinson’s isn’t contagious either.
When Langston—who is now the chief executive officer of the Parkinson’s Institute & Clinical Center, in Sunnyvale, Calif.—gave the immobile pair a popular therapeutic for Parkinson’s patients called levodopa, they sprang back to life almost immediately. Both patients were once again able to walk and move normally.
Around the same time, four similarly afflicted patients popped up at medical centers in the nearby San Francisco area. What all of them had in common, Langston would come to learn, was that they were heroin addicts. And they had all recently shot up with a designer, heroin-like concoction. The drug underworld was about to electrify Parkinson’s research and raise some difficult questions about pesticides.
Around 1980, chemistry-savvy criminals, looking for ways to get around illegal-substance laws, began synthesizing modified versions of illegal and controlled compounds such as LSD and morphine that gave users similar highs. One drug a few of the criminal chemists made was 1-methyl-4-phenyl-propionoxypiperidine (MPPP), a derivative of the painkiller Demerol with five times the potency.
But some of these crooked chemists did sloppy work. To speed up the MPPP synthesis, they increased the reaction temperature, unwittingly generating a dangerous by-product, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP).
Together with Ian Irwin, the director of the Drug Assay Laboratory at Stanford University Hospital, Langston identified MPTP in drug samples recovered from his patients. In a paper the scientists published in the Feb. 25, 1983, issue of Science, they hypothesized that MPTP had caused the addicts’ Parkinson’s-like behaviors (DOI: 10.1126/science.6823561).
About five months after Irwin and Langston’s Science paper came out, researchers at the National Institute of Mental Health confirmed MPTP’s toxicity. Upon repeatedly injecting monkeys with low doses of MPTP, the scientists observed the animals slow down and go rigid (Proc. Natl. Acad. Sci. USA1983,80, 4546). And just as it had helped Carillo and the others, the drug levodopa gave the monkeys back their ability to move freely.
The compound that seemingly crippled at least six people had simultaneously helped generate an animal model of Parkinson’s disease. It also sparked a movement in the research community to look for other substances that might be causing Parkinson’s in the wider population.
In 1984, a research team at the University of California, San Francisco, proved that a metabolite of MPTP called 1-methyl-4-phenylpyridinium (MPP+) was the actual neurotoxic culprit that crippled the addicts (Biochem. Biophys. Res. Commun. 1984,120, 574). Once inside their brains, MPTP got converted by a monoamine oxidase enzyme into MPP+, a nerve cell killer.
Researchers also discovered that MPP+ had been going by another name: cyperquat. During the 1970s, at least one firm, Gulf Oil Chemical Co., was developing cyperquat as an herbicide to protect crops against the weed nutsedge. Although cyperquat isn’t used today, its structurally similar cousin, paraquat, is one of the 25 most commonly used pesticides in the U.S., according to a 2007 survey conducted by the Environmental Protection Agency.
The MPTP saga had put scientists everywhere on high alert to the possibility that pesticides played some role in Parkinson’s.
MOLECULAR DETECTIVE WORK
What motivated Pittsburgh’s Greenamyre to test rotenone in rats in the first place wasn’t the fact that it was a pesticide. Nor was it because of any structural similarity to MPP+. Rotenone, a naturally occurring pesticide derived from the roots of tropical plants, is a much larger molecule than MPP+, with five carbon-based rings in its flavonoid backbone rather than just a biphenyl set of two.
Greenamyre tested rotenone because, like MPP+, it inhibits complex I, a group of proteins in the membrane of mitochondria, cells’ power-generating organelles. When complex I gets blocked, cells can’t produce the chemical energy they need to survive. “We thought it was a good way to test the idea that complex I inhibition would lead to the selective neurodegeneration seen in Parkinson’s,” Greenamyre recalls.
In Parkinson’s disease as well as in the condition afflicting the California addicts, nerve cells in the brain’s substantia nigra that produce the neurotransmitter dopamine die. This stops the flow of dopamine molecules to neurons in another area called the striatum, causing a short circuit in the brain’s ability to tell a person’s limbs to move.
When Greenamyre and his group—then at Emory University—administered rotenone to their rats in the late 1990s, they saw the selective neurodegeneration in the substantia nigra they’d guessed at (Nat. Neurosci. 2000, DOI: 10.1038/81834). What’s most interesting about this nerve cell die-off, Greenamyre says, is that even though rotenone is injected into the rats’ bloodstream and blocks complex I in cells all over the body, the neurons in the substantia nigra are the only ones to show signs of damage. “People don’t yet understand the selectivity of it, but clearly the dopamine neurons in that region are most vulnerable,” he adds.
TRAIL OF CLUES: How Parkinson’s and pesticides got linked
MPP+ also blocks complex I and kills neurons in the substantia nigra, but it’s even more selective because of the way it enters cells. Rotenone, a lipophilic compound, gets inside cells by squeezing through their membranes. MPP+, on the other hand, gets taken up only by dopamine-producing nerve cells. Protein pumps that stud the surface of these neurons transport MPP+ inside, trying to usher back in excess dopamine that’s released when a nerve fires, but getting a lethal compound instead.
But Greenamyre’s rotenone-exposed rats are helping his team and others do more than just learn about the mechanisms behind Parkinson’s disease. “They’re providing proof of concept that exposure to certain classes of chemicals—pesticides being one—can cause selective nerve cell damage,” he says.
Once used for household gardening, rotenone is now applied outdoors only as a piscicide—a chemical that can clear pest fish from a lake or pond. Recognizing rotenone’s toxicity, the U.S. voluntarily restricted its use to fish in 2007.
The same can’t be said about MPP+’s cousin, paraquat. In 2007, about 3 million lb of the chemical was applied to land in the U.S. as a broad-spectrum herbicide. Although the mechanism of how the compound gets into the brain’s nerve cells is still under dispute, a handful of research groups have shown that it does damage to neurons in the substantia nigra—although not nearly as much damage as MPP+.
One reason paraquat causes less damage might be that the compound doesn’t directly bind to complex I, Greenamyre says. Instead, it undergoes a process called redox cycling in cells, in which it forms harmful reactive oxygen species. “Because of the oxidative damage that ensues, complex I ends up getting damaged,” Greenamyre explains, “so paraquat indirectly inhibits complex I,” at least at high, millimolar concentrations.
However, other teams have failed to elicit any nerve cell damage in paraquat-treated mice. Phil Botham, European head of product safety at Syngenta Crop Protection, says he and a team of scientists have gone to great lengths to test a variety of paraquat doses in mice and have observed no neuronal loss in the rodents’ substantia nigra (Neurotoxicol. 2013, DOI: 10.1016/j.neuro.2013.03.005). Syngenta manufactures paraquat.
“After a great deal of animal research, we’ve concluded that paraquat isn’t an adequate model for inducing Parkinson’s syndrome or Parkinson’s disease,” says Tim Pastoor, principal scientist at Syngenta.
Critics also say that the extreme amount of pesticides that get injected into animals in the lab to produce Parkinson’s behaviors is a far cry from how people would be exposed in everyday life. Therefore, researchers have turned to epidemiological studies to get at whether pesticides are triggering the disease outside the lab.
POPULATION SLEUTHS
One of the first surveys to implicate pesticides as a causative agent in Parkinson’s disease was carried out in Canada in the mid-1980s. After hearing of the MPTP hullabaloo, neurologist Ali H. Rajput of the University of Saskatchewan and his team surveyed approximately 20 early-onset Parkinson’s patients—those diagnosed under the age of 40—living in the province. Rajput’s team found that early in life, the patients spent 92% of their time in a rural environment, where they could have been exposed to a “wide range of pesticides” and where they could have drank a lot of potentially contaminated well water (Can. J. Neurol. Sci.1986,13, 312.)
From that modest start, epidemiological studies of the association between pesticides and Parkinson’s took off. During the 1990s, risk factors fluctuated from study to study. Many investigations found that people who worked with pesticides regularly were about two times more likely to have Parkinson’s. Others showed no association.
A lot of these early investigations weren’t very sophisticated, says Beate Ritz, an epidemiologist at the University of California, Los Angeles. Patients would report from memory whether they’d worked with pesticides, so “there was a lot of recall bias,” Ritz says. Plus, she adds, the studies sometimes probed only a yes or no relationship: “Have you ever worked with a pesticide?”
Nowadays, “more and more studies are being done where the exposure information is much higher quality,” says Freya Kamel, an epidemiologist at the National Institute of Environmental Health Sciences, in Research Triangle Park, N.C.
Kamel recently worked with a team including Caroline M. Tanner, the director of clinical research at the Parkinson’s Institute, to poll more than 100 registered pesticide users—mostly farmers—in Iowa and North Carolina. The so-called FAME (Farming & Movement Evaluation) study reported that people who worked with paraquat or rotenone during their lifetimes were two to three times more likely to have Parkinson’s than those never exposed (Environ. Health Perspect. 2011, DOI: 10.1289/ehp.1002839).
Ritz, on the other hand, wants to know whether living and working near farm fields correlates with a higher incidence of Parkinson’s, just as inhaling secondhand smoke affects a nonsmoker’s health.
To probe the issue, in 2011 she surveyed some 700 subjects living in California’s Central Valley, one of the most farmed regions in the world. To participate in her study, Ritz says, “you didn’t have to be a farmer—you could’ve been a firefighter in a firehouse next to a crop field.”
On the basis of subjects’ residential and work addresses, Ritz and her group used California’s Pesticide Use Reporting (PUR) program to estimate individuals’ pesticide exposure over a 25-year period. PUR mandates that farmers report their pesticide use monthly.
By itself, ambient paraquat exposure increased the risk of having Parkinson’s only a small amount, according to Ritz’s results. But people exposed to a combination of paraquat and the fungicides maneb and ziram at their workplaces were three times more likely to have Parkinson’s than people not exposed (Eur. J. Epidemiol. 2011, DOI: 10.1007/s10654-011-9574-5). Ritz believes pesticides may work in concert to lower a person’s molecular defenses.
“Many pesticides are designed to be neurotoxic to pests. Why should we be surprised that they’re neurotoxic to humans?” she asks.
Syngenta disputes the link between paraquat and Parkinson’s. The company has done its own epidemiological work, surveying the death certificates of people who worked in one of its paraquat manufacturing plants, in Widnes, England, between 1961 and 1995. The number of deaths due to Parkinson’s was no higher than that caused by Parkinson’s in the general population, Syngenta found (BMJ Open 2011, DOI: 10.1136/bmjopen-2011-000283).
“From an industry standpoint, it’s somewhat defensible to say, ‘Hey, how do you know it’s really that specific compound?’ when there are thousands of them out there,” says Gary W. Miller, a neurotoxicologist at Emory. “It’s tricky when you do a population study that shows there’s an increased incidence. It’s not like people were exposed only to one chemical.”
One of the main reasons controversy rages in the field is simply because of the nature of epidemiology. “It’s a science of observation,” says Serge Przedborski, a neuroscientist at Columbia University. “Epidemiology cannot give you a link. It can only say, ‘When I see A, I see B.’ ”
Nonetheless, the fact that cigarette smoking causes cancer is pretty much accepted today, says Samuel M. Goldman of the Parkinson’s Institute. It took 40 years of epidemiological and biological work to get there, but connections like that do get made.

Still, “the vast majority of us are not getting Parkinson’s, and the vast majority of people who work with pesticides don’t get Parkinson’s,” Goldman says. “So there’s obviously something else at play.”
That “something,” today’s scientists believe, is genetic susceptibility. Along with Tanner and Kamel, Goldman explored this gene-environment interaction recently by surveying a group of male farmers. The researchers genotyped the participants’ DNA to determine which subjects had mutations in a gene coding for glutathione S-transferase T1. This type of enzyme is responsible for cleansing cells of foreign substances such as pesticides and protecting against oxidative stress.
Men who were exposed to paraquat and who had nonfunctional glutathione S-transferase were 11 times more likely to have Parkinson’s disease than nonexposed men who had functional enzymes (Mov. Disord. 2012, DOI: 10.1002/mds.25216).
Another recent study examined the association of pesticides, Parkinson’s, and mutations to a protein pump called P-glycoprotein. This macromolecule sits on cells lining blood vessels in the brain, defending a person’s gray matter by pushing out molecular intruders.
Agricultural workers in France who were exposed to organochlorine insecticides and who had gene mutations affecting P-glycoprotein’s performance were three to seven times more likely to have Parkinson’s than those who weren’t exposed (Arch. Neurol. 2010, DOI: 10.1001/archneurol.2010.101).
THE PROMISE OF PESTICIDES
While epidemiologists, neurologists, and industrial scientists continue to debate the pesticide-Parkinson’s connection, Pittsburgh’s Greenamyre is thankful for what pesticides offer in the lab. By studying rotenone-treated mice, he says, his group has uncovered certain aspects of human Parkinson’s not previously known. For instance, they uncovered a mechanism by which Parkinson’s patients accumulate iron in the nerve cells of their substantia nigra (Neurobiol. Dis. 2009, DOI: 10.1016/j.nbd.2009.02.009). Excess iron has the ability to generate harmful reactive oxygen species in cells, so the route by which it accumulates is a potential target for treatments.
As far as a link between pesticides and Parkinson’s goes, “I’m highly doubtful that there is any pesticide in the world that is completely safe to all humans exposed to it,” he says. But the neurologist doesn’t think the chemicals should be blindly banned. “We wouldn’t have a modern society and be able to feed the world without them,” he says. What we do need, he argues, is a better understanding of the ones that are most problematic.
To download a pdf of this article, visit http://cenm.ag/pesticides.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter