Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Consumer Products
What is in period products?
A recent New York right-to-know law has led to ingredient disclosures across much of the US. But health advocates say more data are needed on some chemicals’ health effects.
by Cheryl Hogue
October 17, 2022
| A version of this story appeared in
Volume 100, Issue 37

Credit: Shutterstock
In brief
For the first time, consumers in much of the US can find ingredient lists on the packages of most period products. A New York right-to-know law that took effect in 2021 has had nationwide impact, likely because most manufacturers of these products opt for a single package label for US sales. Women’s Voices for the Earth, a women’s health advocacy group that lobbied for the New York disclosure law, is concerned that many chemicals found in pads, panty liners, tampons, and reusable disks and cups haven’t been tested for safety in the permeable skin of the vulva and vagina. The organization also wants manufacturers to specify all chemicals used in product components such as adhesives on pads and panty liners. Makers of period products are opting to speak about the issue through a trade association, which says the companies are committed to providing accurate and useful information to consumers.
The package says the panty liner is made of cotton. But what other substances are in it? Is there an almost-invisible plastic web around that applicator-free tampon? What materials are used to make this reusable, flexible cup designed to collect menstrual fluid?
Tiffany Drummond hadn’t thought to ask these questions.
Drummond, a researcher, educator, and advocate for gynecologic health who posts on social media as Opinionated STEM (science, technology, engineering and mathematics), describes herself as extremely conscious of what she puts into her body. In early 2021, she was searching for information for a YouTube video she was creating about phthalates. These chemicals are often used to make plastics soft and flexible. Studies show that exposure to phthalates can decrease testosterone levels and is associated with adverse effects on the development of children’s nervous systems.
As Drummond searched for information online, she came across a 2013 report from the advocacy group Women’s Voices for the Earth (WVE). That report explored potential health effects of hazardous chemicals in so-called feminine care products such as tampons, pads, wipes, douches, and anti-itch creams designed to be applied to the vulva. She learned that phthalates are sometimes used as an ingredient generically called “fragrance” in these products.
Soon Drummond, who lives in Brooklyn, New York, learned that her state had recently enacted a law that requires manufacturers to include a list of ingredients on period products’ packaging. That statute took effect in 2021 and has led to more ingredient information appearing on these products sold in New York—and even in other states.
WVE and other health advocates say the disclosure of ingredients on period products’ packaging is a huge step forward in learning about the chemicals that many women, girls, and others who menstruate are exposed to in or near their vaginas for 2 to 7 days per month for decades of their lives. Compared with other skin on the body, the skin lining the vulva and vagina is more permeable to some molecules and could therefore lead to greater exposure to chemicals from products it comes in contact with, advocates point out.
“We need to understand what’s in these products that we’re putting into our bodies,” Drummond tells C&EN.
New York law
No federal rules require manufacturers to divulge what’s in period products because the US Food and Drug Administration classifies these items as medical devices. For such items, the FDA requires “appropriate labeling, adverse event reporting and good manufacturing practices,” the industry group Center for Baby and Adult Hygiene Products (BAHP) says. “The FDA has stringent inspection processes in place for all facilities that manufacture them,” BAHP tells C&EN in an email. The group includes major manufacturers of period-care products and their suppliers.
Menstrual pads

Women’s Voices for the Earth compiled these ingredients from online lists and product packages.
Main ingredients:
▸ Absorbent foam, cotton, polyacrylate foam, rayon, superabsorbent polymers, and wood cellulose (or fluff pulp)
Other ingredients:
▸ Aloe barbadensis leaf juice
▸ Aloe vera
▸ Behenyl alcohol
▸ Black 2
▸ Borneol
▸ C12–14 sec-pareth-3
▸ Calcium chloride
▸ Calcium salts of fatty acids
▸ Ceteareth-10 Colorants
▸ Corn mint oil
▸ Deceth-4 phosphate
▸ Diethylhexyl sodium sulfosuccinate
▸ Dioctyl sodium sulfosuccinate
▸ Ditallowethyl hydroxyethylmonium methosulfate
▸ Ethylene brassylate
▸ Ethylene hydrocarbon resin
▸ Ethylene–vinyl acetate copolymer
▸ Ethylene-propylene copolymer
▸ Extract of chamomile
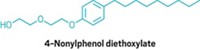
▸ Fragrance
▸ Glycerin
▸ Hot-melt adhesive
▸ Houttuynia cordata oil
▸ Laureth-10
▸ Lavandula angustifolia (English lavender) flower oil
▸ Menthol
▸ Methyl lactate
▸ Naphthenic oil
▸ PEG-10 (polyethylene glycol–10) castor oil
-
▸ PEG-10 dimethicone
▸ PEG-10 laurate
▸ PEG-10 oleate
▸ PEG-11 castor oil
▸ PEG-15 cocoate
▸ PEG monolaurate
▸ Petrolatum
▸ Pigment blue 15
▸ Pigment red 48:2
▸ Pigment red 52:1
▸ Pigment red 57:2
▸ Pigment violet 23
▸ Pigment white 6
▸ Pigment white 21
▸ Pigment yellow 83.
▸ Polyester
▸ Polyethylene
▸ Polyoxyalkylene-substituted chromophore (blue)
▸ Polyoxyethylene lauryl ether phosphate potassium salt
▸ Polyoxyethylene monoallyl ether
▸ Polypropylene
▸ Potassium octadecyl phosphate
▸ Potassium polyoxyethylene lauryl ether phosphate
▸ Rosa damascena flower water
▸ Solvent blue 104
▸ Stearamide DEA
▸ Styrene block copolymer.
▸ Styrene-butadiene copolymer
▸ Synthetic beeswax
▸ Thermoplastic adhesives
▸ Titanium dioxide
▸ Triethyl citrate
▸ Tris(2,4-di-tert-butylphenyl) phosphite
▸ Vinyl acetate
▸ Vitamin E
Menstrual disks

Women’s Voices for the Earth compiled these ingredients from product packages.
Main ingredients:
▸ Medical-grade polymers
Other ingredients:
▸ Antioxidants
▸ Mineral oil
▸ Natural carbon black
Tampons

Women’s Voices for the Earth compiled these ingredients from online lists and product packages.
Main ingredients:
▸ Cotton, rayon
Other ingredients:
-
▸ 4-tert-butylcyclohexyl acetate
▸ α-Isomethyl ionone
▸ Butyl stearate
▸ C. I. disperse blue 60
▸ Carnauba wax
▸ Ceteareth-20
▸ Cetearyl alcohol
▸ Dialkyl sulfosuccinate
▸ Dihexyl fumarate
▸ Dipropylene glycol
▸ Disperse yellow 235
▸ Ether and ester-based oil
▸ Ethoxylated fatty acid esters
▸ Ethyl undecylenate
▸ Ethylene brassylate
▸ Fatty acid polyglycol ester
▸ Fiber finishes
▸ Fragrance
▸ Geraniol
▸ Glycerin
▸ Hexyl cinnamal
▸ Isobornyl cyclohexanol
-
▸ Isobutyl methyl tetrahydropyranol
▸ Limonene
▸ Linalool
▸ Methyl hydrogenated rosinate
▸ Paraffin
▸ PEG-100 (polyethylene glycol–100) stearate
▸ PEG castor oil
▸ PEG cocoate
▸ Pigment white 6
▸ Plant-derived oil
▸ Polyester
▸ Polyethylene
▸ Polymer wax dispersion
▸ Polypropylene
▸ Polysorbate 20
Period underwear

Women’s Voices for the Earth compiled these ingredients from garment labels.
Textile fibers:
Cotton, elastane, Lycra Xtra Life, lyocell, nylon, polyamide, polyester, spandex, Truetex
Other ingredients:
▸ Agion
▸ Carbon
▸ Colorant
▸ Fresh Fix Technology
▸ Hansa SP 1050
▸ Lava XL-N
▸ Polyurethane
Menstrual cups

Women’s Voices for the Earth compiled these ingredients from product packages.
Main ingredients:
▸ Medical-grade silicone
Other ingredients:
▸ Hydroxyl-terminated silicon oil
▸ Organopolysiloxane mixture
▸ Silicon dioxide
- Sources: Women’s Voices for the Earth.
But labeling rules are different for other products that touch the vagina and vulva. The FDA considers douches and intimate wipes to be cosmetics, so makers of these items sold in the US must list ingredients on labels.
New York’s right-to-know law about period product ingredients applies only to products sold in the state, but it is having a nationwide impact, according to a recent report by WVE, which advocated for the statute. For the report, volunteers, including Drummond, went to stores in California, Montana, New York, New Jersey, Pennsylvania, South Carolina, and Wyoming and checked period product packaging. They “found products in other states with ingredient disclosures on the package similar or identical to what is required in New York,” the report says.
Manufacturers of consumer products sold across the US often opt for a single label rather than having separate ones for different jurisdictions. For instance, California requires products to carry warning labels for the presence of certain hazardous chemicals. Manufacturers regularly include these warnings on items sold throughout the country.
But not all US consumers have access to ingredient information for all period products. Some packages found in the WVE field study listed no ingredients. An example is a house brand of pads sold by a grocery chain that doesn’t have outlets in New York. Even a few period products sold in New York lacked ingredient lists. For instance, Drummond found items in deep-discount stores in Brooklyn that were made in Ukraine with packaging information in Turkish.
Some data on ingredients in period products were available online starting in mid-2021. Industry group BAHP hosts a web-based glossary of ingredients used in period-care products that explains why each is used. In addition, many individual brands have lists of ingredients on their websites.
“I never even thought to go to a website” to look up ingredients in period products, Drummond says when told of the glossary. “These companies spend billions of dollars on promotional advertising but never advise women to visit their website for more information.”
Some of the entries in the BAHP online glossary are for specific chemicals, such as octyl potassium phosphate, a surfactant described as enhancing breathability, absorbency, and the ability of a material to repel fluid. Other entries are generic and refer to classes of chemicals, such as quaternized ester amines, which are surfactants that serve the same function as octyl potassium phosphate. The BAHP glossary also lists more than 2 dozen pigments that may be used in period products and disclosed in ingredient lists as “colorant” or “color additive.”
Skin differences
In her work as a gynecologic health advocate, Drummond has counseled people who use feminine-care products to check ingredients and avoid products that contain methylchloroisothiazolinone (often abbreviated as MCI), methylisothiazolinone (abbreviated as MI), parabens, and the quaternary ammonium compound benzethonium chloride. Citing WVE’s work, she says there are few or no publicly available data showing that these chemicals are safe to use on or in the vagina or vulva. Drummond is vigilant about checking ingredients in products used near her genitals because she has and her late mother had serious gynecologic health problems. She avoids chemicals without safety data and urges others to do so.
Women’s health advocates argue that more data are needed to fully assess the risks of ingredients in period products. Some of those ingredients have been tested for use in skin-care cosmetics for the face or hands. But vulvae and vaginas have more permeable skin than most of the rest of the body. That difference makes advocates worry about the safety of these chemicals in period products.
This increased skin permeability is due to a few key factors, according to Margaret Cocks, a board-certified dermatologist in Salt Lake City who specializes in vulval dermatology. The epidermis covering the human head, trunk, and appendages has a layer of granular cells containing the protein keratin. The granular cells are “little extra gatekeepers that protect your skin from the outside world,” Cocks says. The mucosal skin of the vagina and the inner part of the vulva do not have this layer of granular cells, she says.
Unlike the skin of hands and faces, the vagina’s mucosal skin also lacks an impermeable intracellular lipid envelope, making it more permeable to water and soluble proteins, she says. Plus, the vagina is more hydrated, providing greater opportunity for water-soluble substances to penetrate the mucosal skin.
Meanwhile, menstrual pads and panty liners are in constant contact with the vulva. “There’s no separation between that foreign object and the skin,” Cocks says. That situation, called occlusion, boosts chemicals’ ability to penetrate skin, she says.
Alexandra Scranton, director of science and research at WVE, says it’s unclear how period product manufacturers choose ingredients and whether they’ve determined if the exposure of vulvae and vaginas to these chemicals is safe. Cocks says she’s unaware of studies testing cosmetic ingredients on vulval or vaginal tissues—but manufacturing companies may have internal data that they haven’t shared publicly. She notes that skin conditions of the vulva and vagina are an area of medicine that falls beyond the traditional training for dermatologists and gynecologists.
Many patients in Cocks’s weekly vulval dermatology clinic show sensitivity to panty liners, she tells C&EN. Some wear these products daily, and not just during their periods—often to absorb nonmenstrual vaginal discharge. In patients whom Cocks has tested to determine whether they are allergic to liners or pads, liners are often the culprit.
Scranton is concerned about more than just individual chemicals in period products. Some tampons, which are placed inside the vagina, have a plastic sheath covering absorbent material. This gossamer material remains in place during a tampon’s use. According to a recent peer-reviewed study, this thin sheath can release plastic microfibers and billions of nanoplastics in the vagina (Environ. Sci.: Nano 2022 DOI: 10.1039/D1EN00755F). It is unclear whether or how these tiny bits of plastic could affect health when released in a permeable area of the body.
More information needed
In response to the New York statute, manufacturers have provided differing levels of detail in the ingredient lists on period product packaging.
Some companies have disclosed more information on packages about their period products than the industry group BAHP’s web glossary provides, according to WVE’s Scranton. “There were a lot of surprises in the information that we got as a result of the law,” Scranton tells C&EN. One was a longer list of ingredients for each type of product than was formerly posted online, she says.
But ingredients lists on many period products still aren’t as detailed as WVE says they should be to enable consumers to make informed choices. For instance, some stick-on pads that are applied to underpants list “adhesive” as an ingredient rather than specifying the chemicals that constitute the adhesive, the group’s report says. Other examples of listed substances that aren’t chemical specific include “fragrance,” “ink,” “white pigment,” and “surfactant.”
“These are descriptions of the functions of these ingredients but do not allow for identification or understanding of the actual chemical exposure,” the report says. Later, it says, “Listing ingredients by vague descriptors does not allow users to avoid specific allergens or other chemicals of concern to them.”
Such disclosures are feasible, the report says, because some brands of period products identify each chemical plus its function in the item.
WVE found the shortest ingredient lists on menstrual cups and disks. These reusable items, worn internally, are made of flexible material and are designed to collect menstrual flow rather than absorb it, as pads and tampons do. WVE found that several product packages listed only medical-grade silicone.
However, the report says, “Several of the cups we looked at were clearly colored”—for example, some of these products are red or black—yet “no dyes or colorants were ever disclosed,” the report says. Some packages also listed antioxidants and “natural carbon black.”
The WVE report also looked at a relative newcomer to the period-care market—washable, reusable, wicking underwear that is absorbent, and leak resistant. Outer packages of underwear didn’t list specific ingredients. As required of clothing sold in the US, the underwear inside the packages had either labels or tags that listed the fabric types—such as polyester, spandex, polyamide, and cotton. One brand of underwear, Thinx, also listed additives that included a surfactant, an ingredient that counteracts odors, and a silver-based antimicrobial, the report says.
Makers of two brands of period underwear, KT by Knix and Thinx, are facing separate lawsuits claiming that independent tests show their products contain per- and polyfluoroalkyl substances (PFAS). Among the many uses of PFAS, some of these chemicals can make fabrics waterproof and stain resistant. These synthetic compounds are highly resistant to breakdown in the environment, and some are toxic.
Advertisement
Both Thinx, which is majority owned by Kimberly-Clark, and apparel maker Knix say their own tests show their products have no PFAS.
Meanwhile, Cora, a maker of period underwear, is using ingredient disclosure as a marketing tool. Its website boasts that its underwear is “made without toxic chemicals, PFAS, or azo dyes.”
Confidential information
Requirements to disclose period-care ingredients will soon grow. In 2023, California’s Menstrual Products Right to Know Act will take effect. Like the New York statute, the California law requires ingredients be listed on the packages of period products. But the legislation allows manufacturers to withhold the identities of ingredients that are claimed as confidential business information. WVE opposed the California law because of this provision.
The new law raises the question of whether manufacturers will create separate labels to comply with the disclosure laws in New York and California.
Companies that are members of BAHP “are committed to providing accurate and useful information,” the industry group says in its email to C&EN. Variations among labels on period-care products “depend on the individual manufacturers, regulations in the countries where the products are sold, language of the country, and other local determinants.”
Among BAHP members are the two biggest US producers of pads and tampons: Kimberly-Clark, owner of flagship brands Kotex and U by Kotex; and Procter & Gamble, with Always and Tampax. Neither company responded to C&EN’s queries about this article. Other BAHP members include Eastman Chemical, Henkel, and International Paper.
BAHP tells C&EN it is working with state and federal lawmakers in the US “to form clear and consistent labeling guidelines, which provide information to consumers and predictability for product manufacturers.” The industry group adds, “Consistent and clear labeling requirements are important for consumer trust and compliance by product manufacturers.”
Drummond sees ingredient disclosure as essential to helping consumers make informed decisions.
Download a PDF of the print version of this story here.
“We need to be concerned with labels on our period and feminine-care products just as much as we are about labels on the foods we consume,” Drummond says.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter